Agent
Ranaviruses are a particular group of viruses that infect amphibians, although some can infect fish and/or reptiles too. There are several different types of ranavirus. Some are more host-specific than others (i.e. some infect only one type or species of animal, while others can infect multiple species or types of animal). Different species of amphibians differ in their susceptibility to ranavirus infection and/or ranavirus disease (which is called ranavirosis).
Previously, just one group of ranaviruses, referred to as frog virus 3 (FV3) – like viruses, was known to be present in Great Britain and is thought to have been introduced to Great Britain on multiple occasions since the 1980s, most likely from North America. Recent research, however, has identified a second group of ranaviruses in British amphibians, known as common midwife toad virus (CMTV) – like viruses. The origin of these CMTV-like viruses is currently unknown, with a retrospective study dating the first known case of CMTV-associated disease in a British amphibian to 1995.
Species affected
All species of amphibian in Great Britain are considered to be susceptible to ranavirus infection, but the common frog (Rana temporaria) and the common toad (Bufo bufo) are most frequently reported with the disease. There is some evidence to suggest that the common toad might be less susceptible to ranavirosis than the common frog. Generally, tadpoles, metamorphs and adult amphibians are susceptible to ranavirosis, but in Great Britain, adult amphibians appear to be most commonly affected, with tadpole mortality being rarely reported. Ranaviruses that infect amphibians often can also infect fish and/or reptiles.
Signs of disease
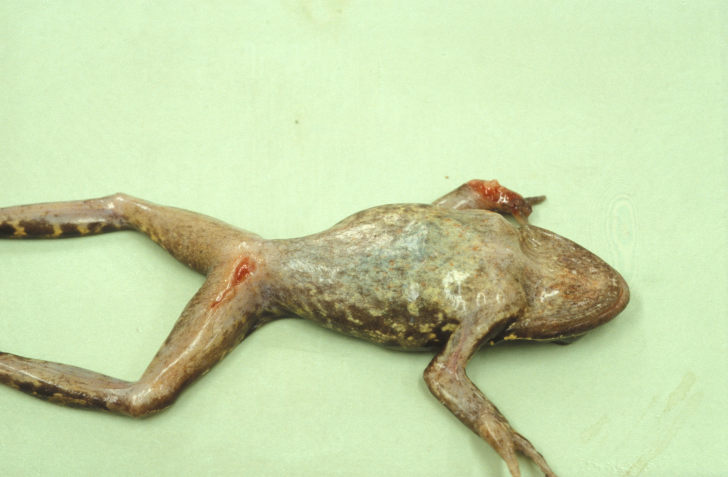
Outbreaks of ranavirosis can vary from the presence of numerous dead and/or sick amphibians (which are often visible in, and surrounding, water bodies), to individual sick animals being seen. Affected adult amphibians may have reddening of the skin, skin ulceration, bloody mucus in the mouth and might pass blood from the rectum; often there is internal bleeding (which is detected on post-mortem examination). Often, large numbers of dead animals are found with no evidence of disease, but these animals invariably have died of internal bleeding. Individual sick animals usually are lethargic and have skin ulceration or loss of digits (fingers and toes), which sometimes progresses to the loss of entire feet (Figure 1).
Figure 1. Common frog (Rana temporaria) with ranavirus disease showing skin ulceration and loss of digits. (Photo credit: Zoological Society of London)
Diseased larval amphibians often have swollen bodies and signs of internal bleeding, such as red patches in the tail or body. Death in susceptible amphibians can occur within a few days following infection or may take several weeks.
Many of the signs of ranavirosis are typical of a disease syndrome which is commonly called “red leg”. Ranaviruses are not the only possible cause of “red leg” in amphibians and other possible causes, such as bacterial infection or normal variation in skin colouration, should be considered.
Whilst ranavirosis can occur at any time of year when amphibians are active, most outbreaks occur during the warmer summer months. Higher ambient temperatures have been shown to be associated with increased frequency and severity of ranavirosis outbreaks. If climate change and warming continues, it is predicted that the severity of ranavirosis outbreaks may increase; that they may occur over a wider geographical range in Great Britain; and with an extended season that could include the spring breeding season and therefore lead to tadpole mortality. Currently, in Great Britain, the disease has only been seen in adult amphibians in the wild, during the summer months.
Although suspected in other species (such as the common toad), the negative effect of ranavirus infection at a population level in Great Britain has only been demonstrated in common frogs in England. Here, ranavirus disease has been shown to cause marked declines, and in some cases local extinctions, of common frog populations at infected sites since the 1990s.
Disease transmission
Ranaviruses are highly infectious and are capable of surviving for extended periods of time in the environment, even in dried material. Ranaviruses can persist in the aquatic environment outside a host for more than two months. Transmission between individuals occurs by indirect and direct routes, and includes exposure to contaminated water or soil, contact with infected individuals, and ingestion of infected tissue.
Ranaviruses can be introduced into new areas via the movement of infected animals, contaminated water or aquatic plants, or on contaminated equipment, such as boots and fishing equipment.
Distribution and origin
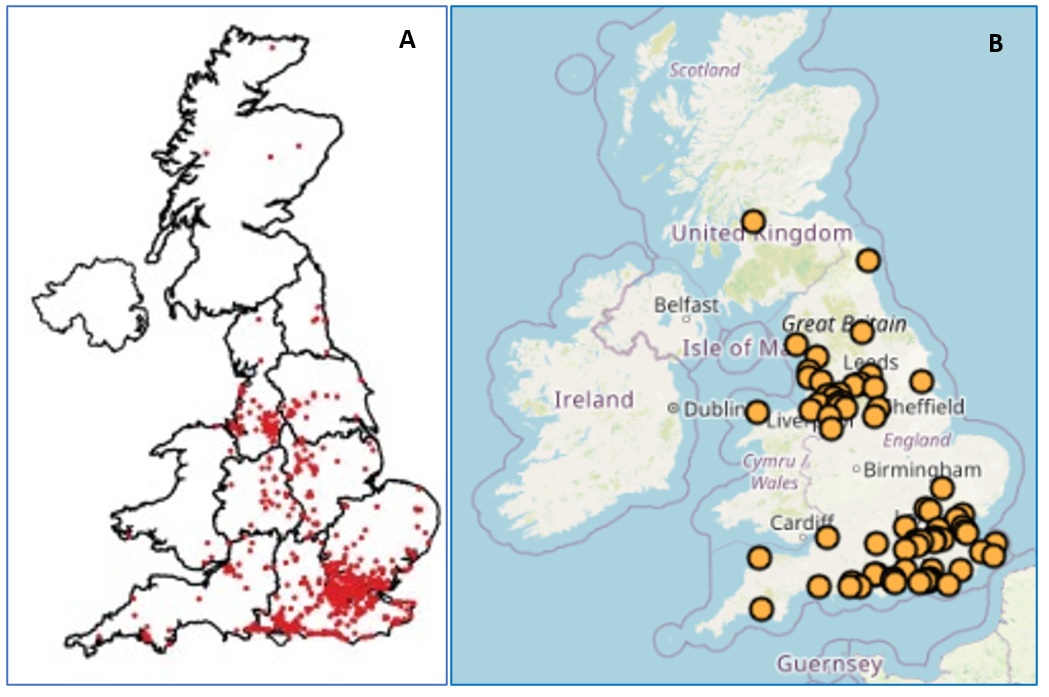
Amphibian ranavirosis is known to occur in many parts of the world, including North America, Australia and Europe. In Europe, we initially discovered the disease in southern and south east England in the early 1990s. Since then, scientists at the Zoological Society of London and Froglife have continued to investigate the emergence and spread of amphibian ranavirosis in Great Britain. The disease is thought to have spread through a combination of natural amphibian dispersal and human-assisted movement of infected animals and contaminated materials. Figure 2 shows the distribution of reports consistent with Ranavirosis in Great Britain since 1992.
Figure 2. Maps showing locations of suspected ranavirosis incidents in Great Britain.
A. Ranavirosis-consistent incidents reported to the Frog Mortality Project (which later merged with Garden Wildlife Health), 1992-2010 (Price et al., 2016).
B. Ranavirosis-consistent incidents reported to the Garden Wildlife Health project, 2013-2020.
Risk to human health
No known risk to human health.
Risk to domestic animal health
Ranaviruses that infect amphibians often can also infect fish and/or reptiles. The main lineage of ranavirus that is known to infect amphibians in Great Britain (FV3-like) is also known to cause lethal disease in pet tortoises (Testudo spp.). It is possible that the virus can cause disease in a variety of fish species, although this has not yet been confirmed in the wild.
Diagnosis
While there are several characteristic features of ranavirosis in amphibians, none of them are specific to this disease. Additionally, amphibians may die of ranavirus infection without any observable signs of disease. This means that the diagnosis of ranavirosis can only be made following post-mortem examination with additional specialist laboratory testing.
If you wish to report finding a dead amphibian, or signs of disease in amphibians, please visit www.gardenwildlifehealth.org. Alternatively, if you have further queries or have no internet access, please call the Garden Wildlife Health vets on 0207 449 6685.
Control and prevention
There are no effective measures known for the treatment or control of ranavirosis. During a disease outbreak, the chances of animals becoming infected should be reduced by removing dead amphibians as soon as possible; the preferred method of disposal being burial as this prevents other animals coming into contact with infected carcasses.
Since there is evidence that higher environmental temperatures lead to increased frequency and severity of ranavirosis outbreaks, the provision of opportunities for amphibians in garden ponds to regulate their body temperature may be helpful in disease control. Dependent on site, options might include shading, log piles, larger ponds, or areas of deeper water within ponds, to provide a range of available ambient temperatures. The chances of introducing ranavirus to a new site can be minimised by avoiding the introduction of potentially-infected material (spawn, tadpoles, amphibians, water or water plants) to new sites and by cleaning and disinfecting boots and equipment that might be used in different ponds or other water bodies.
If you keep captive amphibians, it is important that you ensure good biosecurity practice to minimise risks of virus transmission to wild amphibians. The disease alert document listed below provides information and guidance on what you can do to reduce disease risks to captive and wild amphibians in the UK.
Further information
Garden Wildlife Health (2020). Amphibian disease alert.
https://www.gardenwildlifehealth.org/wp-content/uploads/sites/12/2020/06/Amphibian_Disease_Alert.pdf
Garden Wildlife Health (2020). Guidelines for safe disposal of waste water and other materials from captive amphibian enclosures.
https://www.gardenwildlifehealth.org/wp-content/uploads/sites/12/2020/06/Amphibian_Waste_Disposal_Guidelines.pdf
ARG UK (2017). ARG UK Advice Note 4: Amphibian Disease Precautions: A Guide for UK Fieldworkers. Amphibian and Reptile Groups of the United Kingdom.
https://www.arc-trust.org/amphibian-disease-precautions
World Organisation for Animal Health (OIE) Disease card: infection with ranavirus.
https://www.oie.int/fileadmin/Home/eng/Internationa_Standard_Setting/docs/pdf/Ranavirus_card_final.pdf
World Organisation for Animal Health (OIE) Diagnostic manual for aquatic animal diseases.
https://www.oie.int/doc/ged/D9568.PDF
Scientific publications
Price SJ, Leung WTM, Owen C, Puschendorf R, Sergeant C, Cunningham AA, Balloux F, Garner TWJ, Nicholas RA (2019) Effects of historical and projected climate change on the range and impacts of an emerging wildlife disease. Global Change Biology 25(8):2648-2660 doi.org/10.1111/gcb.14651
Price SJ, Wadia A, Wright ON, Leung WTM, Cunningham AA, Lawson B (2017) Screening of a long-term sample set reveals two Ranavirus lineages in British herpetofauna. PLoS ONE 12(9):e0184768. doi.org/10.1371/journal.pone.0184768
Price SJ, Garner TWJ, Cunningham AA, Langton TES, Nichols RA (2016) Reconstructing the emergence of a lethal infectious disease of wildlife supports a key role for spread through translocations by humans. Proceedings of the Royal Society B 283:20160952. doi.org/10.1098/rspb.2016.0952
North AC, Hodgson DJ, Price SJ, Griffiths AGF (2015) Anthropogenic and Ecological Drivers of Amphibian Disease (Ranavirosis). PLoS ONE 10(6):e0127037. doi.org/10.1371/journal.pone.0127037
Price SJ, Garner TWJ, Nichols RA, Balloux F, Ayres C, Mora-Cabello de Alba A, Bosch J (2014) Collapse of Amphibian Communities Due to an Introduced Ranavirus. Current Biology 24(21):2586-2591 doi.org/10.1016/j.cub.2014.09.028
Teacher, AGF, Cunningham AA, Garner TWJ (2010) Assessing the long-term impact of Ranavirus infection in wild common frog populations. Animal Conservation 13:514-522. doi.org/10.1111/j.1469-1795.2010.00373.x
Teacher AGF, Garner TWJ, Nichols RA (2009) Population genetic patterns suggest a behavioural change in wild common frogs (Rana temporaria) following disease outbreaks (Ranavirus). Molecular Ecology 18:3163-3172. doi.org/10.1111/j.1365-294X.2009.04263.x
Acknowledgements
Current funding for the GWH comes in part from Defra, the Welsh Government and the Animal and Plant Agency (APHA) Diseases of Wildlife Scheme (DoWS) http://apha.defra.gov.uk/vet-gateway/surveillance/seg/wildlife.htm; and from the Esmée Fairbairn Foundation, the Universities Federation for Animal Welfare and the Garfield Weston Foundation.
Disclaimer
This factsheet was produced by Garden Wildlife Health (GWH) for information purposes only. The GWH will not be liable for any loss, damage, cost or expense incurred in or arising by reason of any person relying on information in this fact sheet.
Date of factsheet update:
May 2020